AstraZeneca bronchial asthma drug Fasenra fails a principal aim in section 3 trial for esophagus dysfunction
[ad_1]
Naeblys/iStock by way of Getty Photographs
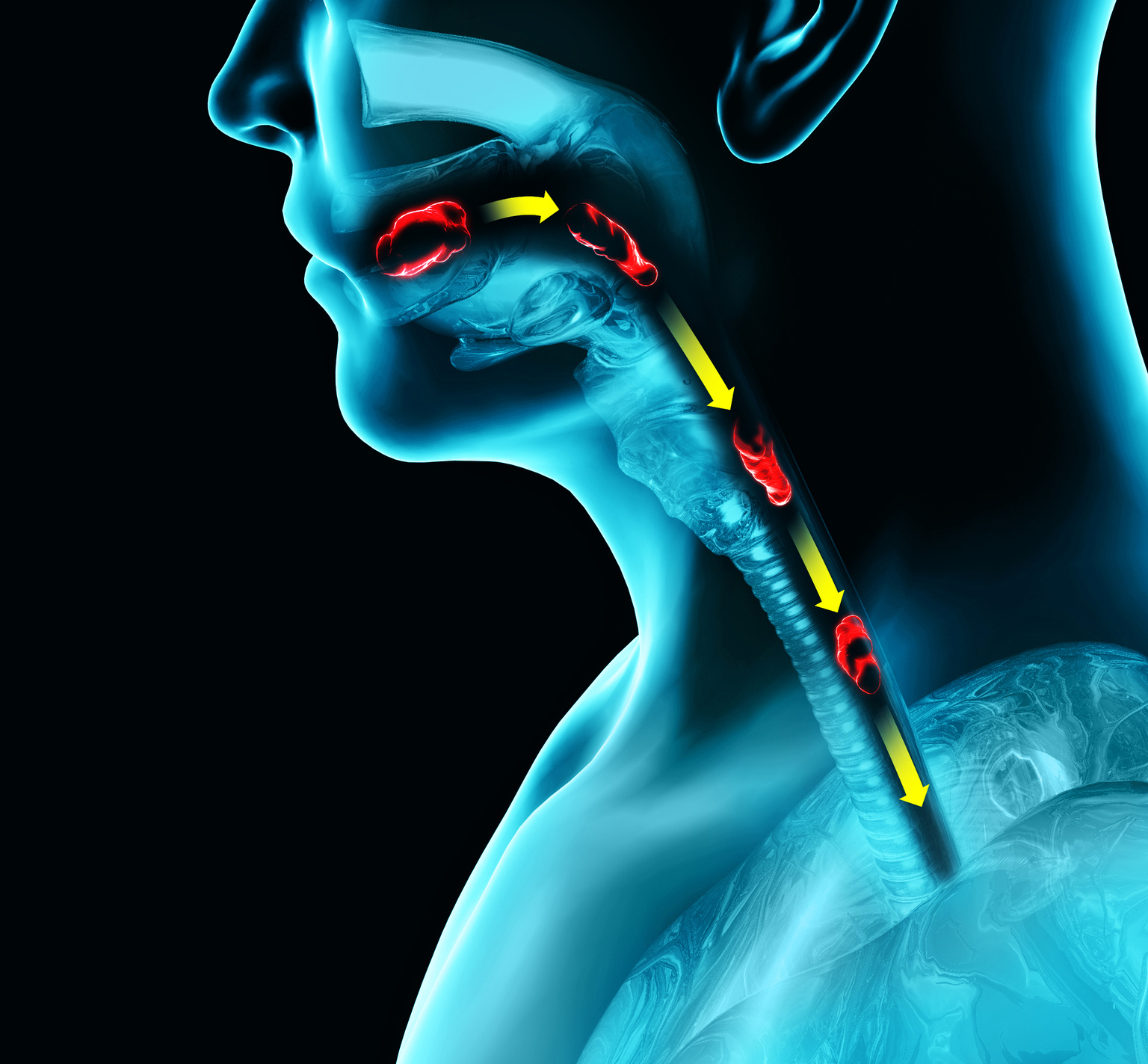
AstraZeneca (NASDAQ:AZN) on Tuesday mentioned its drug Fasenra didn’t meet one of many two principal aim aim of a section 3 trial to deal with sufferers aged 12 to 65 years of age with symptomatic and histologically lively eosinophilic esophagitis (EoE).
EoE is a uncommon, persistent inflammatory illness of the esophagus characterised by the irregular construct up of a sort of white blood cells known as eosinophils within the internal lining of the esophagus. Sufferers expertise problem whereas swallowing (dysphagia), and ache, amongst different signs.
Within the examine, dubbed MESSINA, Fasenra confirmed a statistically vital enchancment in histological illness remission, however not a change in swallowing (dysphagia) signs, in comparison with placebo, in sufferers with EoE aged 12 years or older.
The British pharma large mentioned histological illness remission was measured because the proportion of sufferers with lower than or equal to 6 eosinophils per excessive energy discipline at week 24.
In the meantime, burden of swallowing was evaluated utilizing the patient-reported Dysphagia Symptom Questionnaire (DSQ) and measured as a median change from baseline at week 24, the corporate added.
The examine included 210 sufferers, who acquired both Fasenra (benralizumab) or placebo at four-week intervals.
“The outcomes from the MESSINA Section III trial in eosinophilic esophagitis affirm that Fasenra achieved close to full depletion of tissue eosinophils, in line with its mechanism of motion, nevertheless this didn’t translate into an enchancment in dysphagia signs. We’ll proceed to analyse the whole knowledge set to share with the scientific group,” mentioned Mene Pangalos, govt vice chairman, BioPharmaceuticals R&D, AstraZeneca.
AstraZeneca added that security and tolerability profile for Fasenra within the trial was in line with the identified profile of the drug.
Fasenra is at present authorized as an add-on upkeep remedy for extreme eosinophilic bronchial asthma within the U.S., EU, Japan and sure different international locations.
In March, the U.S. FDA declined to approve Fasenra to deal with sufferers with inadequately managed persistent rhinosinusitis with nasal polyps.
Source link
Recent Posts
Tips on how to Manage Entrance Exam Pressure
Hey there! Are you feeling a little bit overwhelmed with the entrance assessments coming up?…
Top Strategies for Winning at Slot Games
Hey there, fellow slot enthusiast! If you're reading this, chances are you're looking to level…
Typically the Growing Demand for Digital Marketing savvy
Hey there! If you've been considering diving into digital advertising, you're onto something significant. The…
The particular Rise of Dodo69 Video game titles Community
Hey there, fellow video game enthusiast! Have you heard about the hottest buzz in the…
Basement Waterproofing with Epoxy Flooring: A Must-Have for Murrieta Homeowners
Basement waterproofing is a critical account for homeowners in Murrieta, CA, and for good reason.…
Studying the World of Terong123 Games
Here you are in the thrilling universe of Terong123 Games! Imagine walking into a realm…

