Can Abbott keep EPS beat run in Q3 as business faces slowdown in COVID merchandise demand
[ad_1]
Sundry Pictures
Abbott Laboratories (NYSE:ABT) is scheduled to announce Q3 earnings outcomes on Wednesday, October nineteenth, earlier than market open.
The consensus EPS Estimate is $0.94 (-32.9% Y/Y) and the consensus Income Estimate is $9.65B (-11.7% Y/Y).
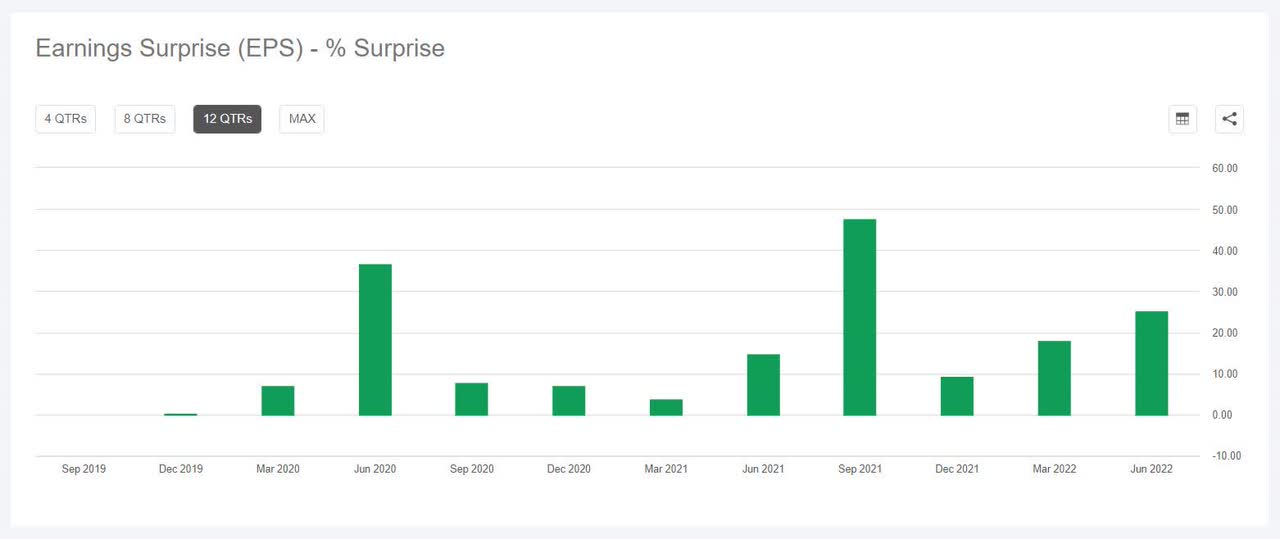
During the last 2 years, ABT has overwhelmed EPS estimates 100% of the time and has overwhelmed income estimates 88% of the time.
During the last 3 months, EPS estimates have seen 3 upward revisions and 13 downward. Income estimates have seen 7 upward revisions and seven downward.
Forward of the corporate’s Q3 outcomes, SA contributor Mike Zaccardi wrote that merchants anticipate a small share value transfer however a big per-share revenue decline.
Abbott’s inventory rose +1.17% on July 20 after Q2 outcomes beat analysts’ estimates and the corporate raised its FY22 steering. Worldwide gross sales from COVID-19 testing exceeded Road forecasts to succeed in $2.3B.
COVID-19:
It could be fascinating to see how the gross sales from COVID merchandise pans out in Q3 and the approaching months. Earlier within the day, Roche reported its Q3 outcomes, whereby group gross sales declined 6% Y/Y, amid decrease COVID-19-related gross sales. Johnson & Johnson additionally noticed ~3% Y/Y fall within the gross sales of its COVID-19 vaccine in Q3.
WHO Director Common Tedros Ghebreyesus hinted in September that the COVID-19 pandemic was not over however its finish was in sight. The U.S. FDA additionally up to date its COVID-19 check coverage and plans to evaluate solely a small variety of new emergency use authorization (EUA) requests for diagnostic checks.
In the meantime, the U.S. authorities deliberate to purchase greater than 100M further at-home speedy COVID checks from native producers regardless of funding constraints forward of a possible improve in demand within the fall and winter. In August, it was reported that the Biden administration was ending a program of offering free at-home COVID-19 checks despatched by mail because of lack of funding.
Child components woes:
The 12 months has seen Abbott mired with points surrounding the corporate’s child components. In keeping with a U.S. FDA report, the shutdown of a key Abbott child components manufacturing plant because of suspected contamination and the nationwide scarcity of product which adopted was because of “confluence of systemic vulnerabilities.”
The corporate restarted manufacturing of its Similac toddler components model in August at its facility in Sturgis, Michigan. Amid the scarcity, Abbott had additionally prolonged rebates for a month via the federal WIC diet program.
Different Information:
Earlier this month, the FDA granted EUA to Abbott’s Alinity m MPXV check for monkeypox. The WHO had declared the monkeypox outbreak a world well being emergency in July.
In September, the FDA issued an alert relating to potential clip lock malfunctions in Abbott’s MitraClip implant machine.
Abbott can be increasing its presence in Eire by constructing a brand new manufacturing plant as a part of €440M funding.
Current earnings Evaluation from our contributors:Abbott Laboratories: Shares Stay Costly Whereas In A Technical Downtrend Forward Of EarningsAbbott Is Doubtless To Beat Q3 Estimates – This is Why I am Nonetheless Not A Purchaser
Source link

